
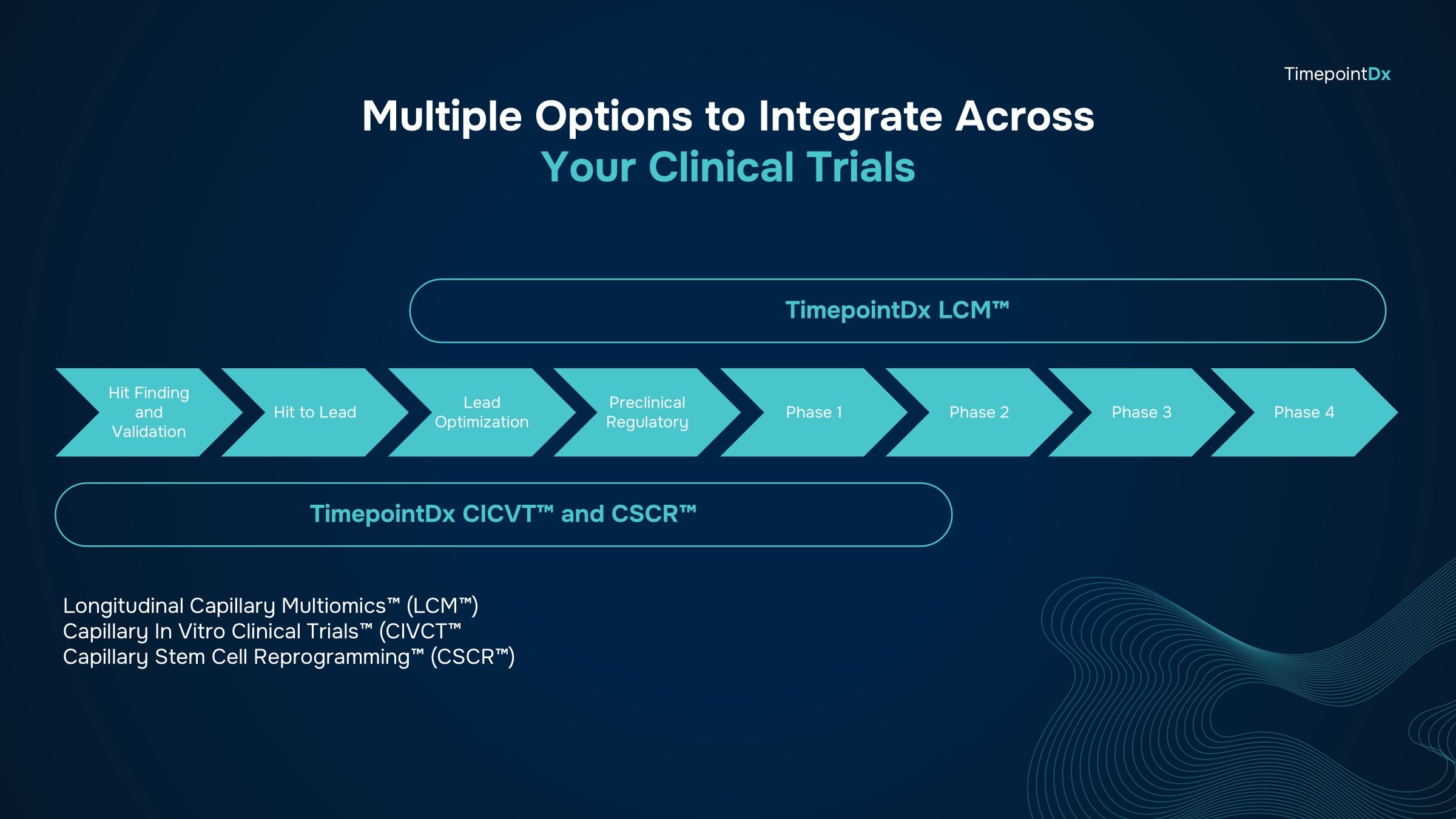
Empowering translational biomarker teams with Longitudinal Capillary Multiomics™

Standardize Your Biomarker Discovery Program Across Your Entire Pipeline via LCM™
-
Preclinical and Discovery
Preclinical asset development and drug discovery via LCM™. Perfect use case for LCM™ in observational studies and more. Gain unbiased hypothesis-generation for drug development.
-
Exploratory
Enhance primary and secondary endpoints with robust biomarker information. Validate exploratory assays in tandem with CDx and IVD development. Discover new pathways of disease and molecular signatures of response.
-
Phase 1-4 + Open Label Extension (OLE) Studies
Enable therapeutic drug monitoring via harmonized LCM™ across all enrolled patients. Drive personalized and precision diagnostics with LCM™ as the biomarker standard of practice.
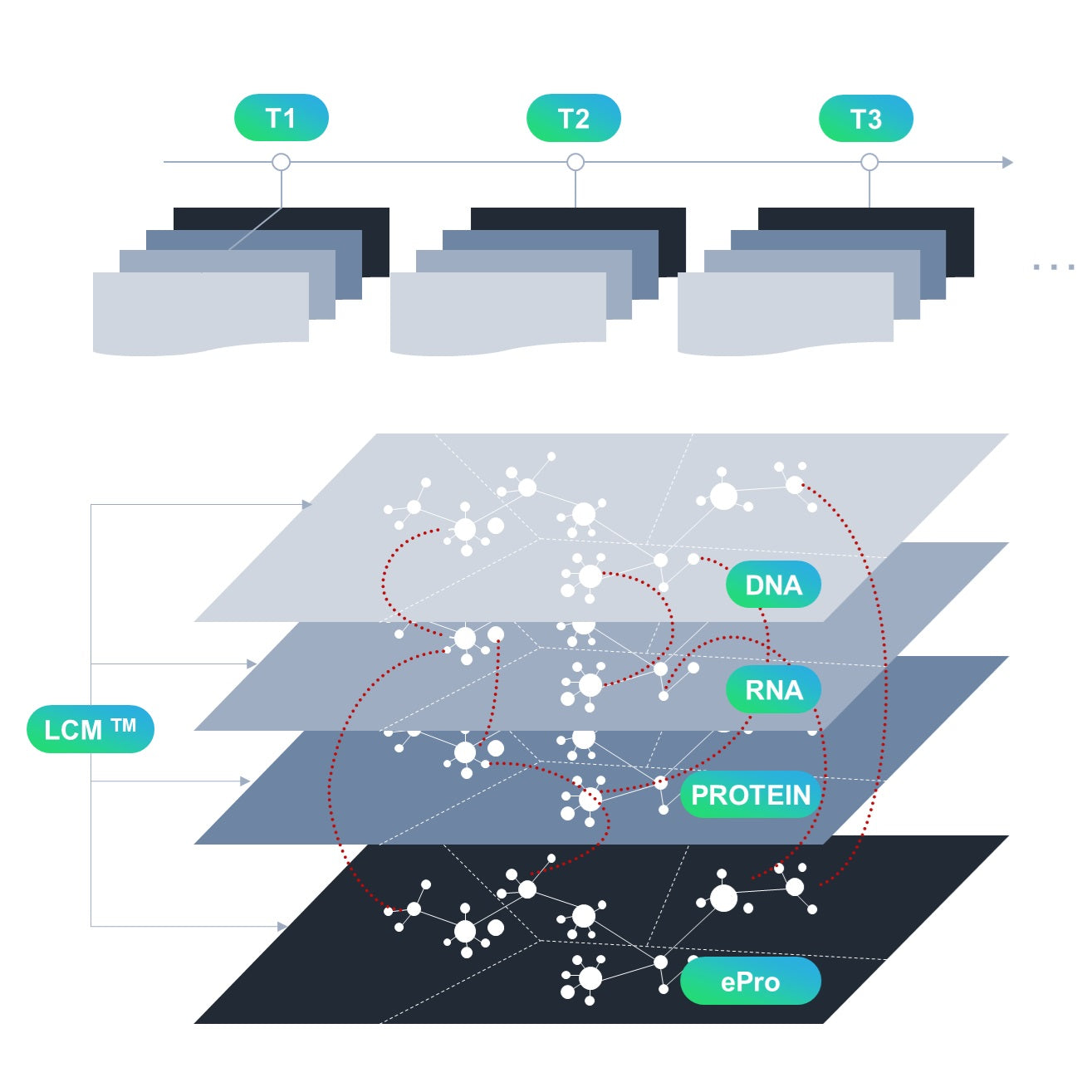
Patient-centric Mixed-methods
True Time-series
By the minute, hourly, daily, weekly, bi-weekly, monthly, quarterly, annually or as desired by your SOA/visit schedule. Drive true time-series exploratory biomarker discovery with point of care blood collection and LCM™.
Multiomics
Maximal Biomarker Data
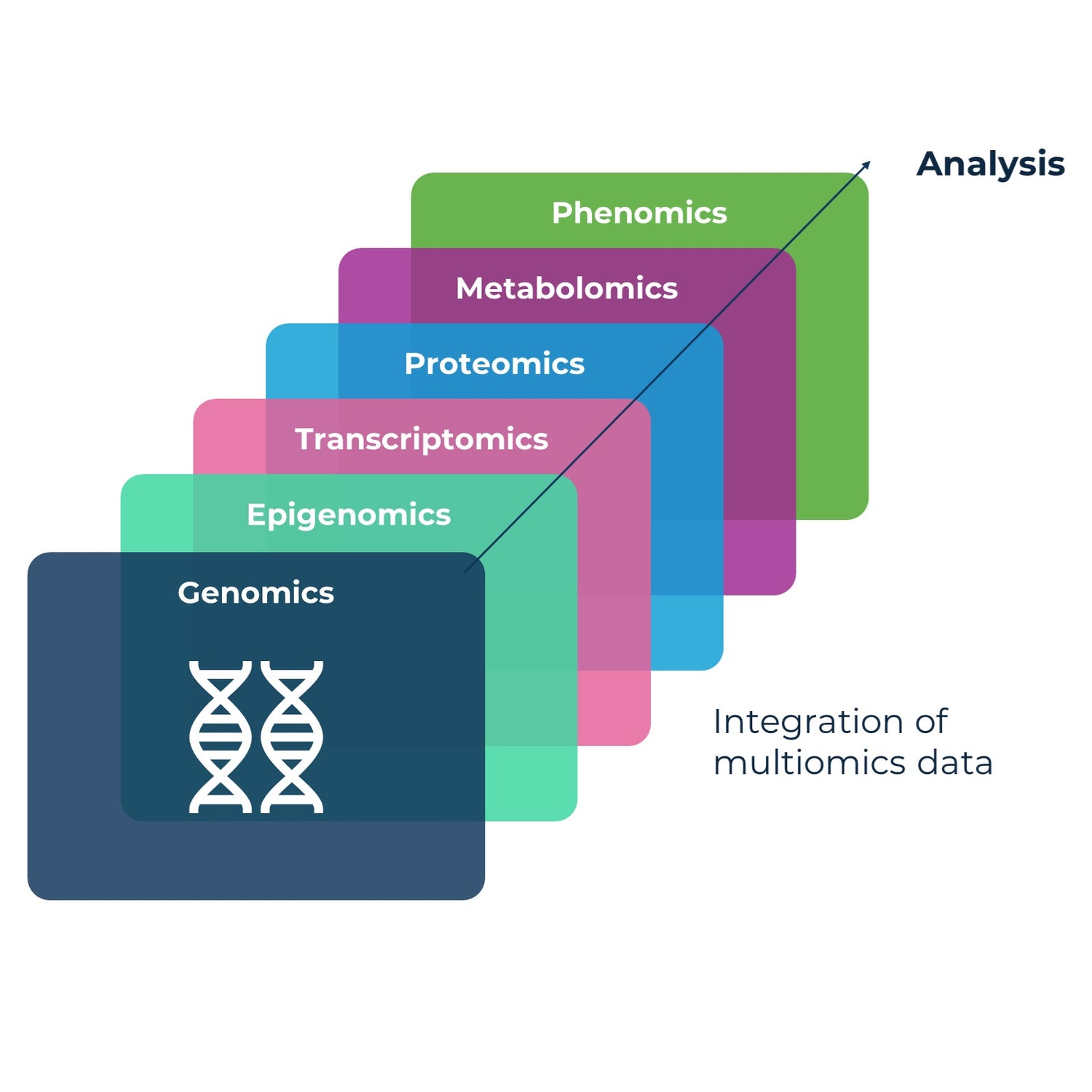
Capture the "central dogma" of biomarkers: genomics (DNA) ► transcriptomics (RNA) ► proteomics (proteins). Every sample goes through the same standardized LCM™ assay for every timepoint collection to generate robust biomarker data.
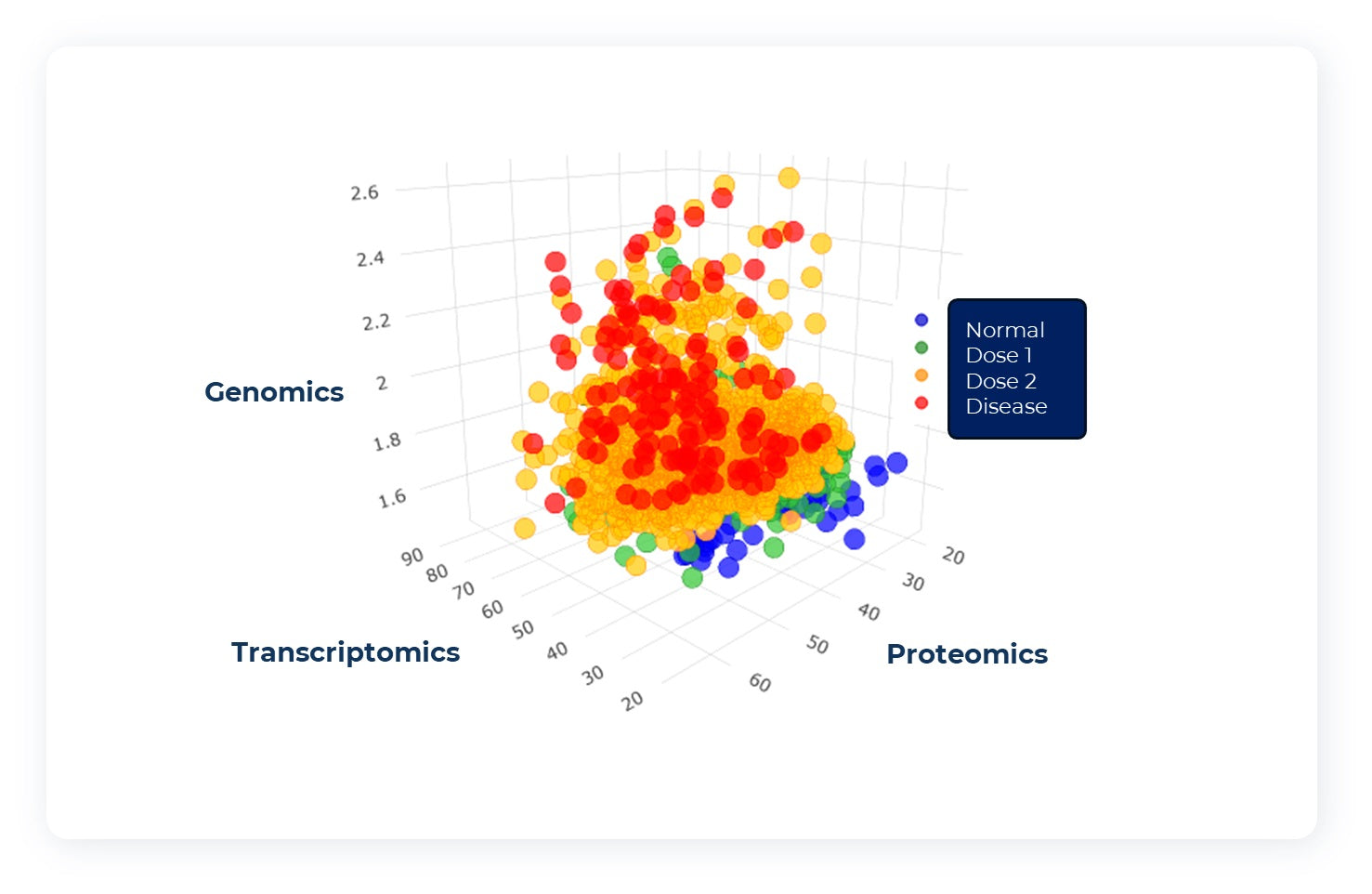
Data Analysis
Unified Bioinformatics
LCM™ is standardized and harmonized, with standard deliverables including BCL, FASTQ, VCF, NPX, excel and more (just a slither of the raw data files that you will received). We can integrate directly with your biomarkers and bioinformatics team. Additionally, TimepointDx can provide our own proprietary algorithmic analysis of LCM™ for every unique patient in your trial.
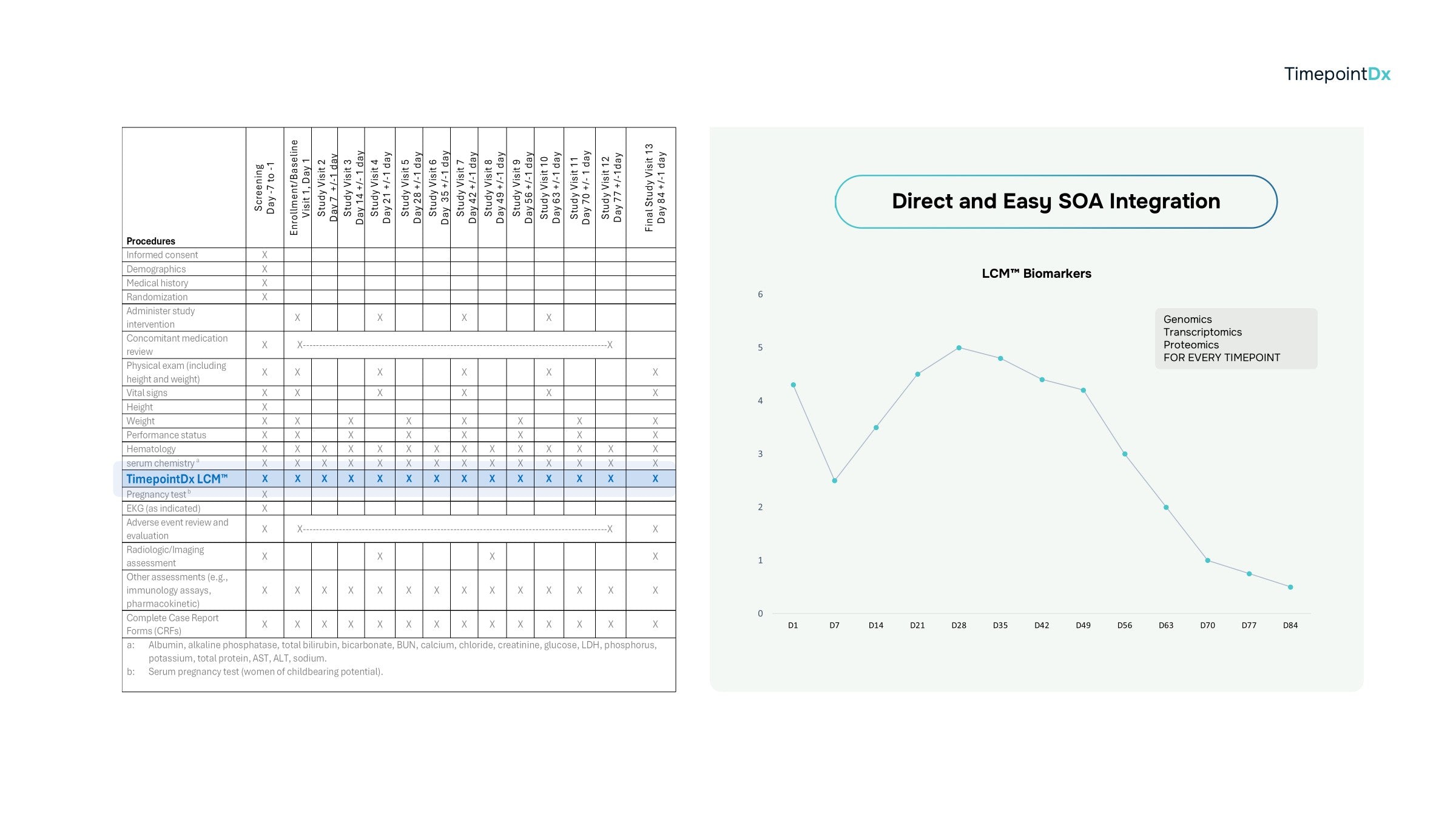
Your Schedule of Assessments (SOA) matched with TimepointDx LCM™ technology.
We know your central lab and have deep relationships. Enjoy seamless integration of LCM™ directly into your SOA, central lab manual, and clinical protocol. We'll take care of the rest for peace of mind.


Benefits for Pharma Trials
Overcome Blood Volume Restrictions
LCM™ overcomes annual blood volume restrictions to enable more sampling events. For perspective, 1 traditional venipuncture timepoint (5-10 ml) is equivalent to 10-20 LCM™ timepoints (~500 ul). Capillary sampling is efficiently convenient.
Patient-centricity (PRO) and User-experience (VOC)
Point of care blood collections devices are virtually painless and simple to use. Patient engagement is enhanced, as trial participants take ownership in their trial-related tasks. TimepointDx adds a layer of patient-reported outcomes (PROs) as part of the collection process to enhance the experience and provide feedback back to cross-functional teams of trials. In other words, we captures VOCs as ePROs and send this information back to you.
Worry-free ICF/ICH Sample Requirements
LCM™ is designated as an "exploratory biomarker" collection and embeds directly into your standardized ICH for exploratory biomarker research. You have the option to exhaust the timepoint sample upon trigger of the LCM™ assay (automatic destruction after test) or retain for up to 20 years via long-term storage until you are ready.
Enhanced Development for Next-Generation Assets
LCM™ exponentiates the R&D for dose-finding and escalation, PKPD, and pathway analysis for next-generation Phase 1 assets. Safety and efficacy studies are enhanced with tighter multiomics timepoints, enabling faster go/no-go decisions for subsequent trial phases.
Standardized Integration Into Entire Pharma Portfolio
LCM™ is easily deployable across your company's entire portfolio of indications and assets. Furthermore, harmonization is achieved with your central lab (or ours). LCM™ is a common standard of practice for Phase 1-3 trials as exploratory biomarkers.
Cost-effective Biomarker Hypothesis Generation
LCM™ is the tool of choice for unbiased hypothesis generation over patient populations. Small capillary blood collections combined with high-throughput processing enables economies of scale for LCM™, passing along the cost-savings to you for exploratory biomarker assessments that are well within your operating budgets. Get faster approvals from your GTL.
Explore Mixed-methods Data Collection Approaches
Whether onsite or remote, ad-hoc collections by patients add significant value for event-driven hypothesis exploration. LCM™ enables timepoints via a combination of mixed-methods data collection and analysis at any time by patients.
Structured and Unstructured Biomarker Data
We provide all raw data as unstructured files. We know your bioinformatics teams' like this and prefer their own pipelines. If needed, TimepointDx offers structured data analysis with our proprietary pipelines. Overall, we don't restrict any raw data and deliver everything in an unbiased format for your AI/ML pipelines.
